Our services
Scientific approach
Our approach consists of, in a first step, checking the available information on the substance(s), by carrying out a bibliographic search and/or by analyzing the data from the studies already available.
Following this first step, we will suggest a strategy to answer the issue.
If studies prove necessary, each test step will be discussed and the progress of the project will be followed in close collaboration with the client.
Explanation of our testing methodology
The aim of the endocrine tests that we perform on any type of sample is multiple:
- to detect possible endocrine activities
- to measure effects on a living organism
- to identify and understand mechanisms of action
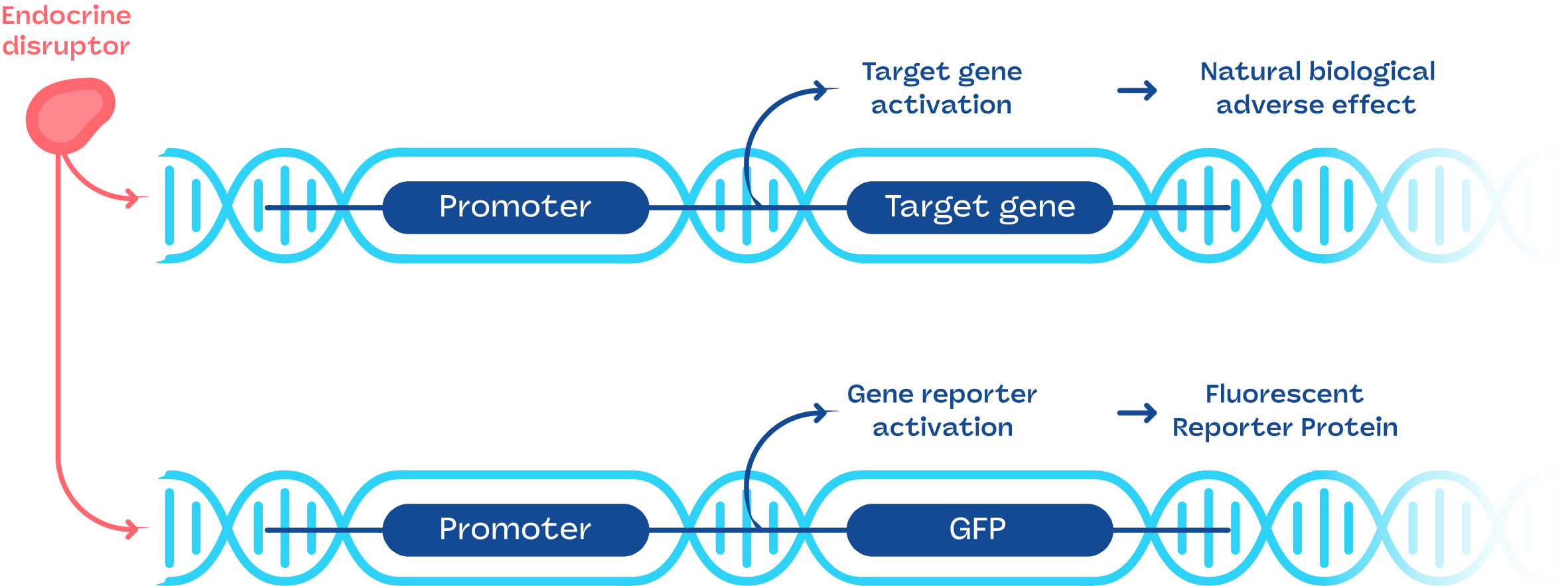
The technology used to achieve this is as follows:
The hormonal mechanisms at these very early stages are identical to those of adult animals and are preserved throughout the evolution of species up to humans.
In order to remain at the ethical stages, we use Medaka eggs (fish from asian ricefields) and Xenopus eggs (amphibians). Just after hatching, the test item is added to the test media, and is exposed to hatched eggs for between 24 and 72 hours.
Eggs harbor a genetic construct composed of the promoter of a naturally occurring target gene driving expression of a fluorescent gene (GFP).
This allows measurement of the level of endocrine activity induced by the tested sample, the level of fluorescence measured is proportional to the effect.

Interview with our team of experts
Why choose a whole organism performed in vitro?
Only an assessment of endocrine activity on an entire organism can provide information on the entire signaling pathway, and thus minimize the risk of false positives or false negatives that could occur with a receptor based approach. However, the challenge was to create models which combine whole organism testing and compliance with ethical issues
What are the main advantages of this technology?
The hormonal mechanisms at this early stage are similar to those of adult stages. Moreover they are preserved through the evolution of species including human beings. In order to quantify the hormonal activity, we use a reporter based on the expression of green fluorescent protein which reveals the endocrine effect. Our choice in using this technology is that we can measure the natural reactions of early life stage organisms without modifying their physiology or their genomic mechanisms
Which endocrine axes should be tested (estrogenic, androgenic, thyroid)?
Several endocrine axes could be potentially impacted by endocrine disruptors. But at this stage, European regulation is focused on three hormonal pathways for which criteria of disruption could be established: Estrogenic, Androgenic (with steroidogenesis) and Thyroid. Defining a testing strategy will then depend on the existing data. If you have none or a lack of information from existing data, we may recommend to investigate the 3 axes. However, based on indications suggesting an endocrine effect, you may want to explore a specific mechanism using a specific model or even define a tailored-made protocol which would allow elucidating the mode of action. This could contribute to the conclusive additional data required by regulatory authorities.
It is important to keep in mind that there is a high degree of cross-talk between the different axes.
Can one test identify an endocrine disruptor?
No, endocrine disruptor identification is hazard based and requires conclusive data to establish the plausible link between the mode of action and an adverse outcome. Neither endocrine activity nor an adverse outcome are sufficient to identify an endocrine disruptor. Our aim is to provide you with data contributing to a weight of evidence approach, which will allow you to make a decision and adapt your testing strategy
What are the communication rules regarding Endocrine Disruption?
Actually there are 2 points in order to correctly answer this question.
1st point is regulatory.
Product claims and consumer communication are very much regulated. Only “authorized” claims may appear on products / media related to the products. So in the absence of a regulatory framework for endocrine disruption labeling, it is recommended to avoid any mention on the products or any advertisement. For any question, you may contact the authorities to get their opinion.
2nd point is scientific.
It would be incorrect to say that a product is “without endocrine disruptors” because variation in endocrine signaling is inherent to living organisms. Our endocrine equilibrium is constantly modified by our environment or by contact with foods or natural or chemical substances. It is therefore very difficult to guarantee the absence of endocrine risk. To eliminate the danger that an endocrine mechanism may lead to a harmful effect, it is necessary to have a large data set. Often several tests and a review of literature data are needed to properly evaluate a substance. The question is particularly complex as interactions between the substances in a product or a mixture can distort the endocrine evaluation. This is why, concerning communication, it is important to show pedagogy and caution.
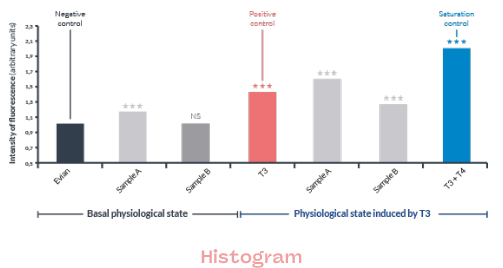
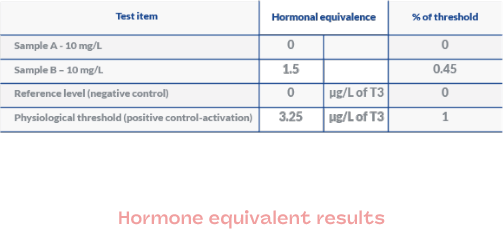
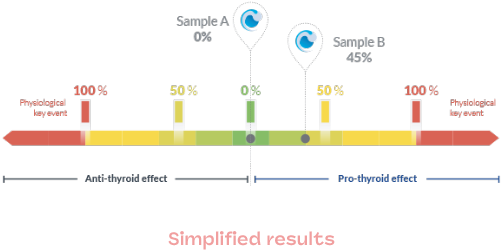
Results at a glance
In order to facilitate understanding of the results and to adapt each report as much as possible to its end use, we can deliver results on several levels:
- Hormonal equivalence
- Raw data
- Histograms
- Images
- Simplified data
This depends on the planned use: regulatory focus, R&D, evaluation of your raw materials and finished products, portfolio screening, …
In addition to the testing report, we will help you interpreting the results